Discovery of Electron :
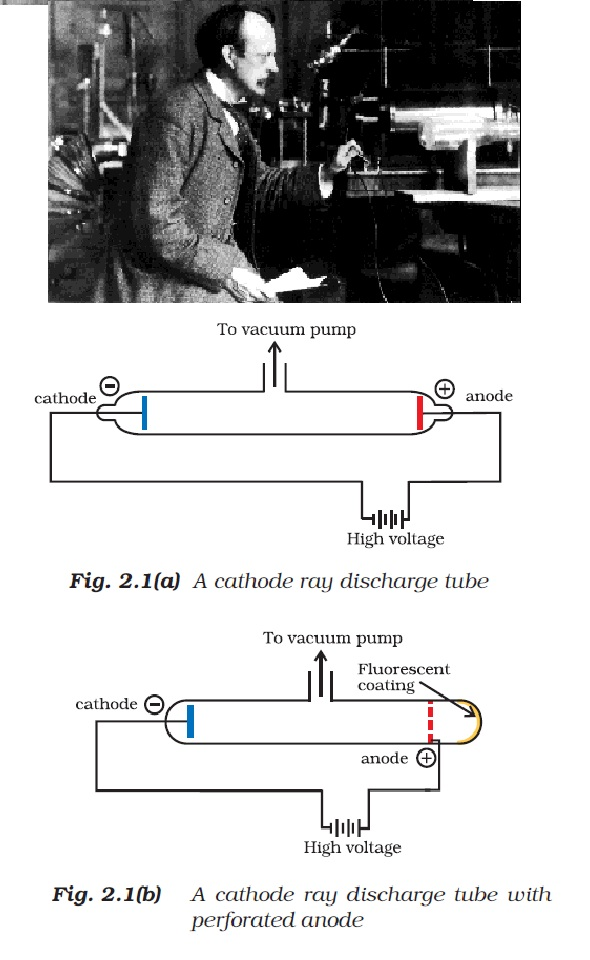
Faraday began to study electrical discharge in partially evacuated tubes, known as cathode ray discharge tubes.
See fig2.1(a) and fig.2.1(b).
`=>` A cathode ray tube is made of glass containing two thin pieces of metal, called electrodes, sealed in it.
`=>` The electrical discharge through the gases could be observed only at very low pressures and at very high voltages. The pressure of different gases could be adjusted by evacuation.
`=>` When sufficiently high voltage is applied across the electrodes, current starts flowing through a stream of particles moving in the tube from the negative electrode (cathode) to the positive electrode (anode). These were called cathode rays or cathode ray particles.
`=>` The flow of current from cathode to anode was further checked by making a hole in the anode and coating the tube behind anode with phosphorescent material zinc sulphide. When these rays, after passing through anode, strike the zinc sulphide coating, a bright spot on the coating is developed(same thing happens in a television set)
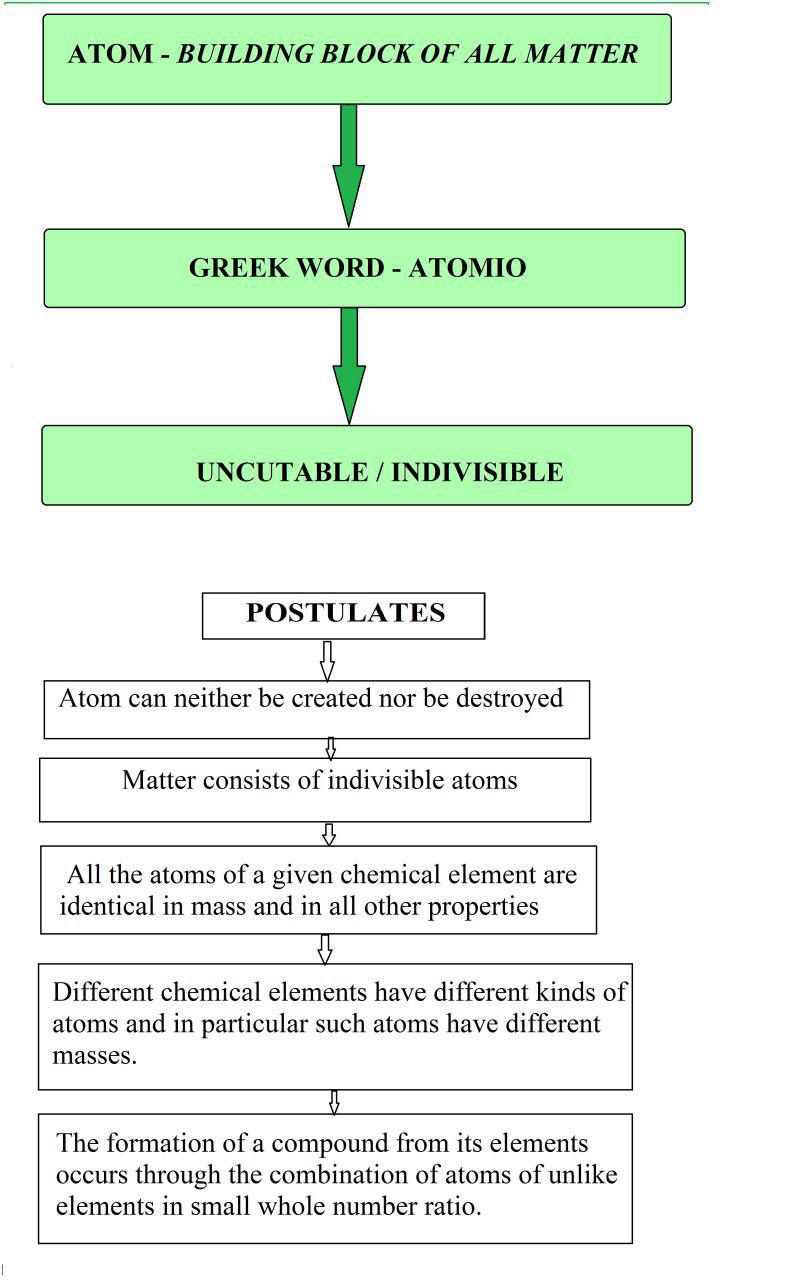
The results of these experiments are summarised below :
(i) The cathode rays start from cathode and move towards the anode.
(ii) These rays are invisible but their behaviour can be observed with the help of certain kind of materials (fluorescent or phosphorescent) which glow when hit by them. Television picture tubes are cathode ray tubes.
(iii) In the absence of electrical or magnetic field, these rays travel in straight lines.
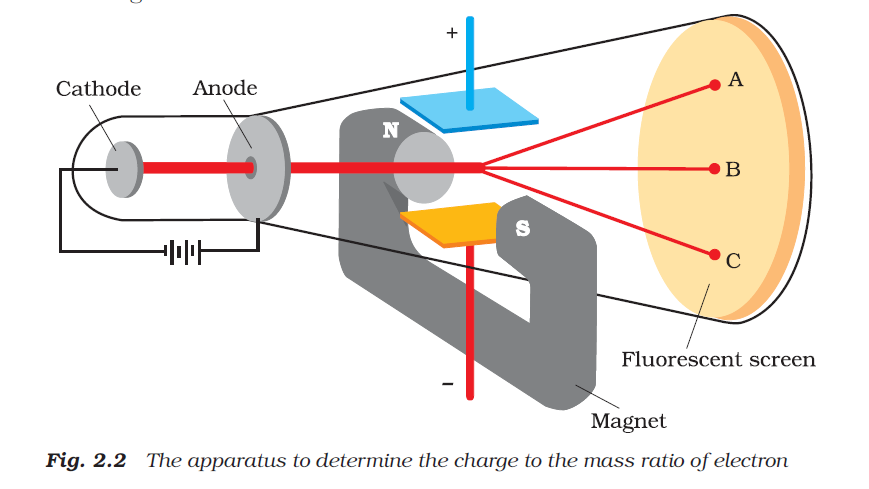
(iv) In the presence of electrical or magnetic field, the behaviour of cathode rays are similar to that expected from negatively charged particles, suggesting that the cathode rays consist of negatively charged particles, called electrons.
(v) The characteristics of cathode rays (electrons) do not depend upon the material of electrodes and the nature of the gas present in the cathode ray tube.
Thus, we can conclude that electrons are basic constituent of all the atoms.
Discovery of Electron :
Faraday began to study electrical discharge in partially evacuated tubes, known as cathode ray discharge tubes.
See fig2.1(a) and fig.2.1(b).
`=>` A cathode ray tube is made of glass containing two thin pieces of metal, called electrodes, sealed in it.
`=>` The electrical discharge through the gases could be observed only at very low pressures and at very high voltages. The pressure of different gases could be adjusted by evacuation.
`=>` When sufficiently high voltage is applied across the electrodes, current starts flowing through a stream of particles moving in the tube from the negative electrode (cathode) to the positive electrode (anode). These were called cathode rays or cathode ray particles.
`=>` The flow of current from cathode to anode was further checked by making a hole in the anode and coating the tube behind anode with phosphorescent material zinc sulphide. When these rays, after passing through anode, strike the zinc sulphide coating, a bright spot on the coating is developed(same thing happens in a television set)
The results of these experiments are summarised below :
(i) The cathode rays start from cathode and move towards the anode.
(ii) These rays are invisible but their behaviour can be observed with the help of certain kind of materials (fluorescent or phosphorescent) which glow when hit by them. Television picture tubes are cathode ray tubes.
(iii) In the absence of electrical or magnetic field, these rays travel in straight lines.
(iv) In the presence of electrical or magnetic field, the behaviour of cathode rays are similar to that expected from negatively charged particles, suggesting that the cathode rays consist of negatively charged particles, called electrons.
(v) The characteristics of cathode rays (electrons) do not depend upon the material of electrodes and the nature of the gas present in the cathode ray tube.
Thus, we can conclude that electrons are basic constituent of all the atoms.